KNEE
- GENUTECH DCF Surgical Technique (ESP)
- GENUTECH DCF Surgical Technique (ENG)
- GENUTECH DCF Express Surgical Technique (ESP)
- GENUTECH DCF Surgery Video (ESP)
- GENUTECH CCK Surgical Technique (ESP)
- GENUTECH CCK Surgical Technique (ENG)
- GENUTECH CCK Surgery Video (ESP)
- KNEE ARTHROPLASTY Patients Guide (ESP)
HIP
- AUSTIN MOORE Surgical Technique (ESP)
- AUSTIN MOORE Surgical Technique (ENG)
- THOMPSON Reference catalogue (ESP)
- THOMPSON Reference catalogue (ENG)
- FEMORAL HEAD Reference catalogue (ESP)
- FEMORAL HEADS Reference catalogue (ENG)
- BIARTICULAR HEAD Surgical Technique (ESP)
- BIARTICULAR HEAD Surgical Technique (ENG)
- ENDOCEPHALIC HEAD Reference catalogue (ESP)
- ENDOCEPHALIC HEAD Reference catalogue (ENG)
- MÜLLER CUP Surgical Technique (ESP)
- MÜLLER CUP Surgical Technique (ENG)
- QUARTER CUP Surgical Technique (ESP)
- QUARTER CUP Surgical Technique (ENG)
- SELF-LOCKING STEMS Surgical Technique (ESP)
- KAREY PRIMARY STEMS Surgical Technique (ESP)
- KAREY REVISION STEMS Surgical Technique (ESP)
- KAREY PRIMARY STEMS Surgical Technique (ENG)
- KAREY REVISION STEMS Surgical Technique (ENG)
- HIP ARTHROPLASTY Patients guide (ESP)
TRAUMA
OTHERS
- PULSE LAVAGE Reference Catalogue (ESP)
- PULSE LAVAGE Reference catalogue (ENG)
- BONE CEMENT MIXING BOWL Catalogue (ESP)
- BONE CEMENT MIXING BOWL Catalogue (ENG)
- C-PLUG Reference Catalogue (ESP)
- C-PLUG Reference catalogue (ENG)
- BONE CEMENT GUN Catalogue (ESP)
- MIXING SYRINGE Catalogue (ENG)
- BONE CEMENT Reference Catalogue (ESP)
- BONE CEMENT Reference Catalogue (ENG)
- ORCEM BONE CEMENT 1&3 Reference Catalogue (ENG)
- ORCEM BONE CEMENT 1G&3G Reference Catalogue (ENG)
- SURGINIBS Bone Substitute Catalogue (ESP)
- SURGINIBS Bone Substitute Catalogue (ENG)
- SURGIBONE Bone Filling Catalogue (ESP)
- Catalogue bone filling SURGIBONE (ENG)
Bone cement characteristics
In 1958, the very first total hip endoprosthesis implant was performed using PMMA bone cement. Today, over 60 years later, the use of bone cement is a common procedure in hip and knee arthroplasties.
In addition to the technical and chemical properties of different types of bone cements, the functional behaviour of a bone cement is an important aspect to consider when selecting a good cement.
The current composition of the Surgival cement range is backed by extensive experience, acquired over many years, of the main types of cements available on the market. It offers excellent quality without missing out on recognised properties of proven components and complies with the specifications of ISO Standard 5833.
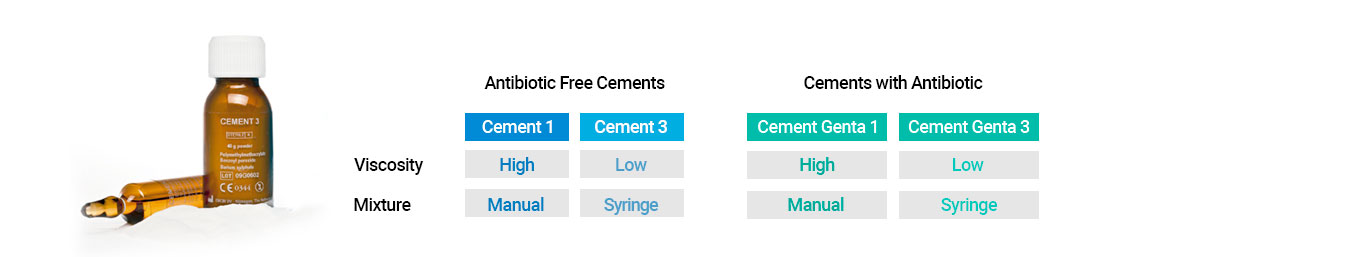
The Surgival cement line offers four types of bone cement:
Specifications:
- Ease of use: Powder and liquid in a single sterile container. The powder is supplied in a bottle to make pouring easier and the liquid phase is supplied in an ampoule.
- Radiopaque agent: barium sulphate.
- Low setting temperature: The maximum exothermic temperature of Surgival cements is between 67°C (Genta 1) and 79°C (Genta 3), well below the 90°C limit established in ISO standard 5833.
- Excellent intrusion for effective fixation: Intrusion for Genta Cement 1 is 7.5 mm, and 8 mm for Cement 1. The ISO standard stipulates that intrusion must not be less than 2 mm.
- Consolidated and proven results: more than 10 years of clinical experience and over 200,000 successful cases.
- Genta Cement 1 and Genta Cement 3 are enriched with Gentamicin (1.2%) to guarantee effective protection against infection.
Composition:
| LIQUID | Cement 1 | Cement 3 | Cement Genta 1 | Cement Genta 3 |
|---|---|---|---|---|
| Content (g) | 14,4 | 16,4 | 14,4 | 16,4 |
| Methyl methacrylate (% of total content) | 84,4 | 84,4 | 84,4 | 84,4 |
| Butyl methacrylate (% of total content) | 13,2 | 13,2 | 13,2 | 13,2 |
| N,N-Dimethyl-p-toluidine (% of total content) | 2,4 | 2,4 | 2,4 | 2,4 |
| Hydroquinone (ppm) | 20 | 20 | 20 | 20 |
| POWDER | Cement 1 | Cement 3 | Genta Cemen 1 | Genta Cement 3 |
|---|---|---|---|---|
| Content (g) | 40 | 40 | 40,8 | 40,8 |
| Poly(methyl methacrylate) (% of total content) | 87,6 | 87,3 | 86,5 | 86,2 |
| Benzoyl peroxide (% of total content) | 2,4 | 2,7 | 2,4 | 2,7 |
| Barium sulphate (% of total content) | 10 | 10 | 9,9 | 9,9 |
| Gentamicin sulphate (% of total content) | - | - | 1,2 | 1,2 |
Uses:
The Cement 1 and the Cement Genta 1, with high viscosity, are recommended for manual application for fixation of cups, total, hybrid or unicondylar knee replacements, shoulder and glenoid prostheses.
The Cement 3 and the Cement Genta 3, with low viscosity, are recommended for syringe application for intramedullary fixation (femur, humerus) and small joints (ulna, radius).

